Now there is more hope, outside of very expensive COVID vaccines, for the Philippines to recover from the pandemic. With new antibodies drugs, given emergency use applications by the Food and Drug Administration, those infected with the virus can now be assured of more medical options from hospitals.
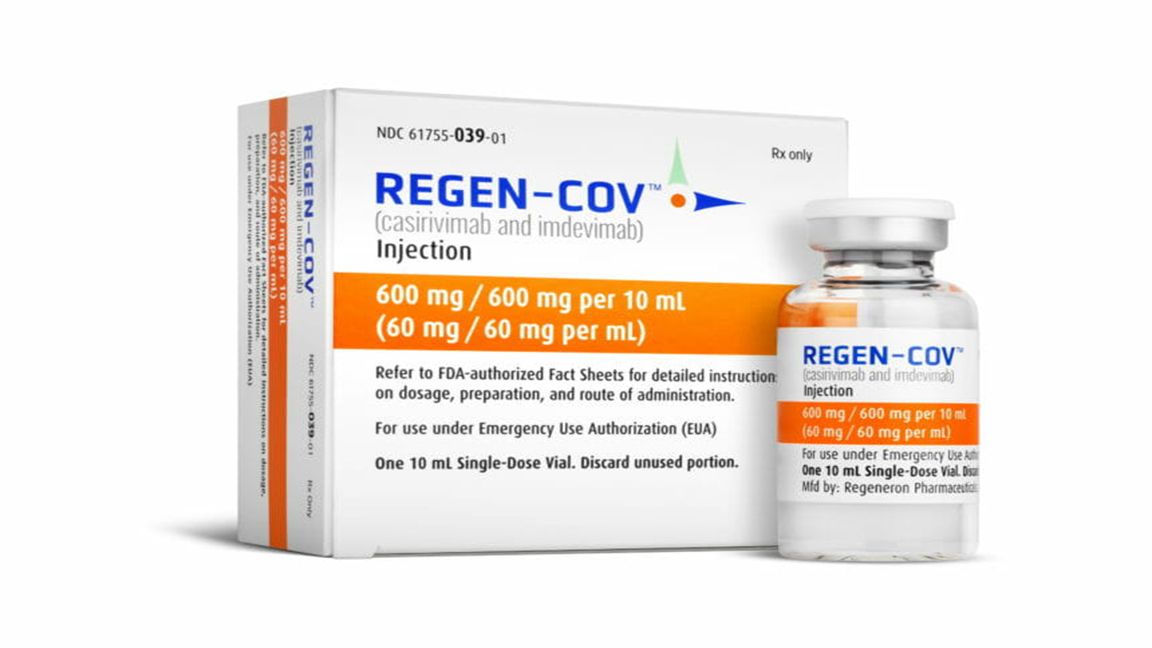
THE issuance of emergency use application to Ronapreve (either by injection or infusion) for mild to moderate COVID infection will augment the government’s treatment option to fight the coronavirus.
Food and Drug Administrator Eric Domingo said Ronapreve, a combination of two monoclonal antibodies-- casirivimab and imdevimab-- was granted EUA on October 1, said a Business Mirror story.
It will be used to treat mild to moderate Covid-19 for patients 12 years old and above.
Ronapreve is intended for people at high risk of developing severe Covid-19.
Also, it can be given to those already showing symptoms or those exposed to someone infected.
Domingo said that since it is combination of two antibodies, it is fighting the infection and should be given during the early part of the illness.
Partnership
Meanwhile, Faberco Life Sciences Inc. (Faberco), appointed distributor of Molnupiravir, the first oral anti-viral drug clinically proven to reduce the risk of Covid-19 hospitalization or death in the Philippines, has partnered with RiteMed Philippines, Inc. (RiteMed) for the latter to distribute the oral drug to hospitals, medical institutions, and treatment sites in the Philippines.
Once compassionate special permits (CSP) are filed with and approved by the Philippine FDA, Molnupiravir may be available in the country as early as next month. They can be accessed initially through hospitals and other health-care facilities.
“We are confident that through the distribution channels of RiteMed, Molnupiravir will reach health-care facilities throughout the country faster, giving more Filipinos access to this life-saving drug the soonest possible time,” said Kishore Hemlani, founder of Faberco.
RiteMed president Jose Maria A. Ochave said the partnership with Faberco puts RiteMed in a good position to “help more Filipinos survive the pandemic and is aligned with our mission to provide access to essential medicines by partnering with like-minded doctors and health-care facilities.”
Faberco was appointed Philippine distributor of Aurobindo Pharma Ltd., a partner manufacturer of Merck & Co. (known as MSD outside the United States and Canada), which developed the Molnupiravir 200mg capsule.
The initial batches of Molnupiravir could be available in the Philippines by the second week of November, subject to regulatory approvals.
FDA’s Domingo said the oral anti-viral drug can be used by doctors and hospitals for Covid treatment via the CSP.
The FDA allows the use of investigational drugs—or drugs not yet registered or in the process of registration—if they are covered by CSP.
An analysis of 775 patients in the US found that only 7.3 percent of those given Molnupiravir were hospitalized, compared to the 14.1 percent of patients who were given a placebo or dummy pill.
There were also no reports of deaths in the Molnupiravir group, whereas eight patients given a placebo in the trial later died of Covid-19.
Unlike most Covid vaccines, which target the spike protein on the outside of the virus, Molnupiravir works by targeting an enzyme the virus uses to make copies of itself.
Merck noted that this should make Molnupiravir equally effective against new variants of the virus as it evolves in the future.
The US drugmaker said its results were so positive that outside monitors had asked to stop the trial early. It said it would apply for Emergency Use Authorization for the drug in the US as soon as possible.
Tags: #antibodies, #cornavirusandvariants, #COVID19, #PhlFDA, #EUA