VACCINES are supposed to heal not kill.
Although health authorities here and abroad are trying their best to downplay and in some cases directly cover-up Covid-19 vaccine-related deaths, there was no denying that people are dying or getting sick after receiving their shots.
24 deaths
Over the weekend, the Food and Drug Administration (FDA) admitted that out of the more than one million Filipinos already vaccinated against Covid-19, 24 people had died.
However, as expected FDA Director General Eric Domingo was quick to explain that most of the reported deaths were unrelated to vaccination.
“Although nakakalungkot po na may nakita tayong mga ganitong kaso, ang evaluation natin…most of it (fatalities) are not related to vaccination,” Domingo said during a press briefing.
As it is, despite the red flags related to vaccination, Domingo just like the other health authorities around the world was quick to defend the efficacy of the vaccines.
It is also worth noting that aside from the reported deaths, more than 31,000 experienced adverse events after immunizations (AEFIs) from the combined total of those injected with AstraZeneca and Sinovac.
Fatalities in other countries
If these vaccines are indeed safe, then why is it that there were also reports of deaths after vaccinations in Norway, Hong Kong, United States and many other countries?
In Norway, 143 mostly elderly people died after receiving their vaccines even as Hong Kong and the US also reported cases of post vaccination deaths.
In Manitoba, Canada, 10 people have died from Covid-19, after receiving at least one dose of the vaccine.
Better than vaccines
With all these reported deaths and incidents of severe side effects in many parts of the world, many people including health experts are wondering why most governments are still pushing hard for use of the hastily created vaccines.
Maybe it’s about time that health authorities look for other better and safer alternative treatments for coronavirus that has been wreaking havoc on our lives for more than a year already.
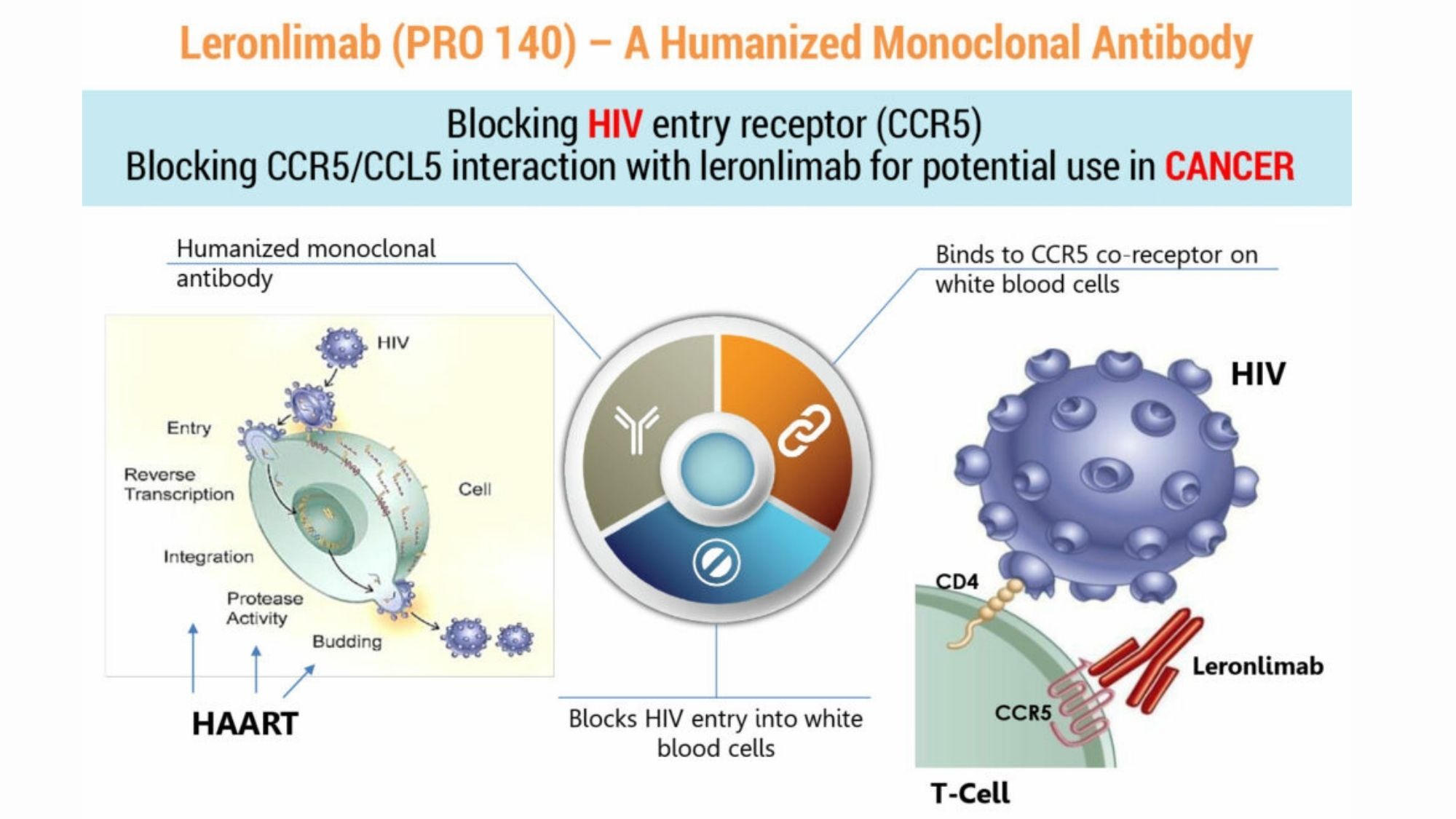
And one of these better replacement for dangerous vaccines is Leronlimab, which despite its reputation as one of the most potent and best drugs ever discovered remains, largely ignored and placed in the backburner by world health authorities particularly the US FDA.
Leronlimab is humanized IgG4 monoclonal antibody (mAb), and based on reports could reduce the amount of inflammation in the lungs caused by coronavirus, helping the patient with his breathing problems by clearing his air passage.
How Leronlimab works?
In essence, if you become infected with coronavirus, your natural immune system sends useful immune chemicals to the affected areas of the lungs to help fight the infection, which is good because this is how you recover from an infection.
However, in some people there is an overreaction by the immune system causing the lungs to become very inflamed, resulting in severe breathing problems and in some cases even death.
Leronlimab works to reduce this rush of immune chemicals to the affected area, and this means there is less inflammation and damage to the lungs.
The actual way it does this is by blocking a protein on the white blood cells called the CCR5 receptor - which is why it is called a CCR5 antagonist (blocker).
The aim of Leronlimab is to block the CCR5 receptor, then the body’s immune system will be able to fight the coronavirus, without the issue of excessive inflammation and other problems.
Big pharmas vs Leronlimab
Certainly, if the claims of people treated by this drug were true, then Leronlimab is indeed better than vaccines.
The catch, however, is that just like Ivermectin, Leronlimab is yet to be approved for use by the US and the local FDA as well as other health agencies in the world.
The reason? Big pharmaceutical firms are reportedly blocking the approval of Leronlimab as treatment not only against Covid-19 but also of cancer and HIV infection.
As it is, Leronlimab is an investigational drug currently being studied for the treatment of HIV infection as well as cancer.
In the past eight years, Leronlimab has not had one adverse reaction and is considered a safe treatment.
However, the US Food and Drug Administration continues to pose roadblocks as it tries to get approval for widespread use.
No need for vaccines
Industry observers believe that if the drug is approved, big pharmaceutical companies will lose billions of dollars as Leronlimab will eliminate the need for other medications particularly Covid-19 vaccines.
Given this fact, there has been constant attempts through the years to make it impossible for the drug to be approved for widespread use.
After all, the biopharmaceutical industry is responsible for providing the FDA with 75 percent of its funding for drug reviews.
This means that for them to not lose funding, they must continue to turn a blind eye to Leronlimab despite its success in treating illnesses such as HIV, cancer, and now, Covid-19.
Erap’s case
Recently, Senator Jinggoy Estrada said that his 84-year-old father, former President Joseph Ejercito Estrada, was treated with Leronlimab after he contracted Covid-19.
Although Mr. Estrada refused to give full credit to Leronlimab for the recovery of President Erap, research shows that it decreases mortality by up to 82 percent among critically-ill Covid-19 patients after two weeks.
Patients who received Leronlimab were 5x more likely to be alive at the end of day 14 than those who received standard hospital care.
400 percent improvement
Critically-ill Covid-19 patients are those receiving invasive mechanical ventilation (IMV).
The administration of the drug also showed a 400% improvement in ranking on the 7-point ordinal scale used to measure clinical status in conjunction to standard care of critically-ill patients.
For the select few who were given the go signal to use the drug in the treatment of Covid-19, it has worked wonders – but what about the ordinary citizens who do not have access to this drug?
Compassionate use
In the Philippines, the FDA granted a compassionate special permit (CSP) to two doctors for the use of Leronlimab to treat COVID-19 patients.
However, they refused to say who these patients are to protect their privacy.
According to the FDA, only institutions or doctors in charge of patients being treated are allowed to request for a CSP. These doctors and institutions should also take full responsibility for the use of Leronlimab.
On top of this, doctors should inform patients that the drug is currently in the investigational status, and that the doctors need to provide a report of the outcomes for each patient given the product.
Supplemental drug
Big pharmaceutical companies would definitely discount using Leronlimab as an effective drug against Covid-19, especially since it would diminish the need for their own medicines.
Moderate greed
However, what these pharmas don’t realize is that Leronlimab works best when supported with other commonly used Covid-19 treatments such as dexamethasone.
Therefore, if these companies would just moderate their greed, their products may actually be used to supplement Leronlimab, and this combination could easily save thousands of lives infected by Covid-19.
FDA must act now
If Leronlimab and also Ivermectin are really that good in treating coronavirus infections, then what’s keeping FDA too long in approving their use for infected patients?
Once FDA approves the use of Leronlimab, the government’s public health care system will improve significantly and fast.
So, it’s about time that the FDA acts swiftly and grant the permit needed for its mass production and authorize its use against the virus.
Certainly, it’s a better replacement for ineffective vaccines that have been causing a lot of pain and anguish to suffering patients. (With report from Monica Otayza)